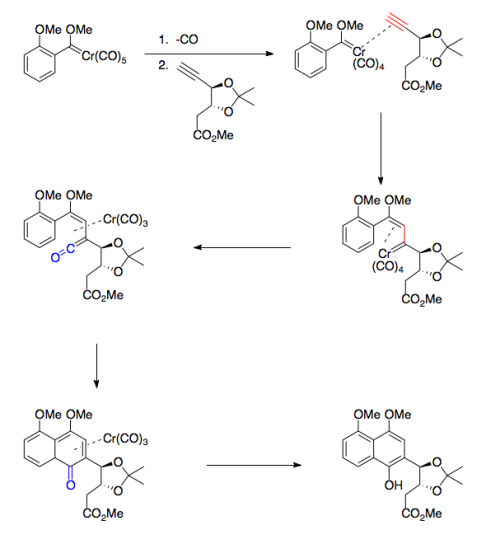
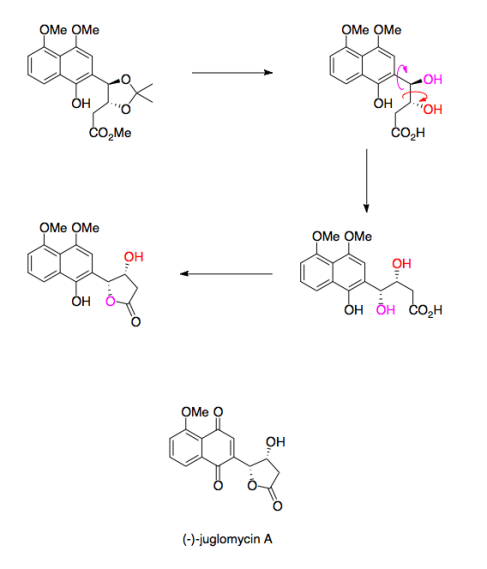
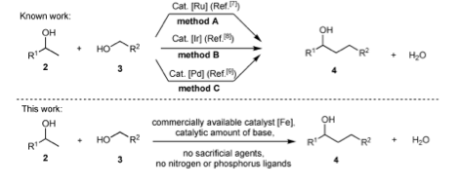
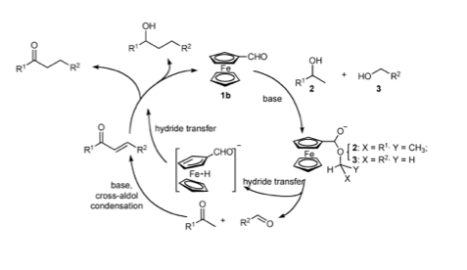
Your Current job: I’m a professor at a small, selective liberal arts college in Pennsylvania. We have seven faculty in the department and my teaching load is mostly organic chemistry.
What you do in a standard work day: Most of my time is spent with students. Organic chemistry is mainly a service course (of the 100 or so students who take organic, only about 8 – 10 will be chemistry majors) so I spend a lot of time helping students who don’t have an innate affinity for the subject. I spend a lot of time trying to develop new ways of conveying the beauty of organic chemistry. Sometimes they work, sometimes they don’t (sounds like chemistry to me!). I also often get to work with students in the lab. An ever-expanding portion of my time is spent on administrative tasks, advising, etc.
What kind of schooling / training / experience helped you get there: I graduated from a (different) small, selective liberal arts college with a BS in chemistry and then earned a PhD from a research university with a concentration in synthetic organometallic chemistry. Two years of a post-doc followed and then a two-year position as a visiting assistant professor at a (different again!) small, selective liberal arts college. (My wife likes to tell friends that I walked on to a college campus as a student in 1976 and have never left.) My “life plan” was to get a position in a smaller research university and happily spend my life with a smallish research group. Reality showed that I was much better suited to a teaching position than a research position and, well, here I am!
How does chemistry inform your work? For most of my work, chemistry is everything. For the non-teaching/non-research portion of my work, training as a scientist allows me to look at problems and issues dispassionately and come to reasonable conclusions. This can be useful in an environment where many of my colleagues operate on the principle that the argument is everything. I prefer to do the experiment.
Finally, a unique, interesting, or funny anecdote about your career: It’s probably the story of how I realized that a teaching career was the right pathway for me. Nearing the end of my two-year visiting assistant professor stint, I was out for a walk around town with my wife and infant son. We happened to go past the department chair’s house and he was having a picnic for his chemistry class, which consisted mainly of students who had been in my organic classes the previous two years. When the students saw us walking by, they all ran from the chair’s yard down to the sidewalk and spent the next 15 minutes talking to my wife, telling us how cute the baby was, etc. Somewhere in the middle of this, probably about the same time the chair wandered down to see where his class had gone, it dawned on me that perhaps I was meant to work with undergraduates rather then spending my time writing grant proposal after grant proposal. Bottom line: sometimes you have to listen to what the world is trying to tell you.